(Ventilators, Sleep and Respiratory Care Devices)
Context:
1) The PE-PUR foam may degrade into particulates, which may enter the device’s air pathway and be ingested or inhaled by the user.
2) The PE-PUR foam may emit certain volatile organic chemicals (VOCs).
Philips Respironics has received several global complaints regarding the presence of black debris/particles within the air pathway circuit (extending from the device outlet, humidifier, tubing, and mask).
Potential side effects: Philips Respironics has received reports of headache, upper airway irritation, cough, chest tightness and sinus infection. In animal trials, some of the volatile chemicals have been shown to be a possible carcinogenic risk. The degradation may be accelerated by unapproved cleaning methods (e.g. ozone). Philips Respironics is conducting testing to better assess the potential short and long-term patient health risks related to these issues.
Links to affected devices: Please click on the link(s) below for a list of devices involved, Philips Respironics most up to date statement & relevant HSE & HPRA information:
Philips Respironics: https://www..ie/healthcare/e/sleep/communications/src-update#section_2
HSE: https://www.hse.ie/eng/services/news/newsfeatures/advice-philips-respironics-devices/
HPRA: http://www.hpra.ie/homepage/medical-devices/special-topics/philips-sleep-and-respiratory-care- devices
HSE National Incident Management Team: The HSE National Incident Management Team (NIMT) is responsible for assessing the impact of this issue for patients in Ireland. This team includes representatives from a range of stakeholder groups including the Health Products Regulatory Authority (HPRA), clinicians, representatives from the private healthcare sector and patient advocacy groups. Since notification of the FSN, the NIMT has extensively engaged with both Philips Respironics and the relevant distributors / service providers in Ireland to ensure that all potentially impacted users of these devices are identified and informed of the field safety notices.
The NIMT, via the HPRA, remains in active dialogue with Philips Respironics and other European and international regulators in relation to the roll out of an appropriate corrective action plan to address this issue and an assessment of the potential toxicological risks in relation to any potential long-term health impacts. This clinical guidance will help inform the appropriate corrective action for Paediatric patients.
Who is this Clinical Guidance for:
This Clinical guidance is has been devised to support clinicians in communicating with and managing the following patient groups using an affected device:
1. Paediatric patients (aged 0-16 years) & their primary caregiver
2. Patients over 16 years, who have yet to transition to adult services, & their primary caregiver
It is based on the limited information received to date and is subject to change as more information becomes available. This guidance refers to Paediatric patients. Separate guidance for the management of Adult Patients is available on the HSE website (link above).
Key Principles for Patient & Primary Care Giver Communication & Management:
- All affected patients will be informed that they have a device that is affected by this Field Safety Notice (FSN). The supplier of the affected device, either Philips Respironics or a relevant Irish distributor/service provider, is undertaking this communication.
- Based on the current information supplied by Philips Respironics, the Health Service Executive (HSE) recommends that all affected patients should stay on their current device until it is repaired or replaced by the relevant distributor/service provider.
- The HSE NIMT have liaised with Philips & relevant suppliers/distributors to assess and prioritise patient groups for the replacement/repair programme.
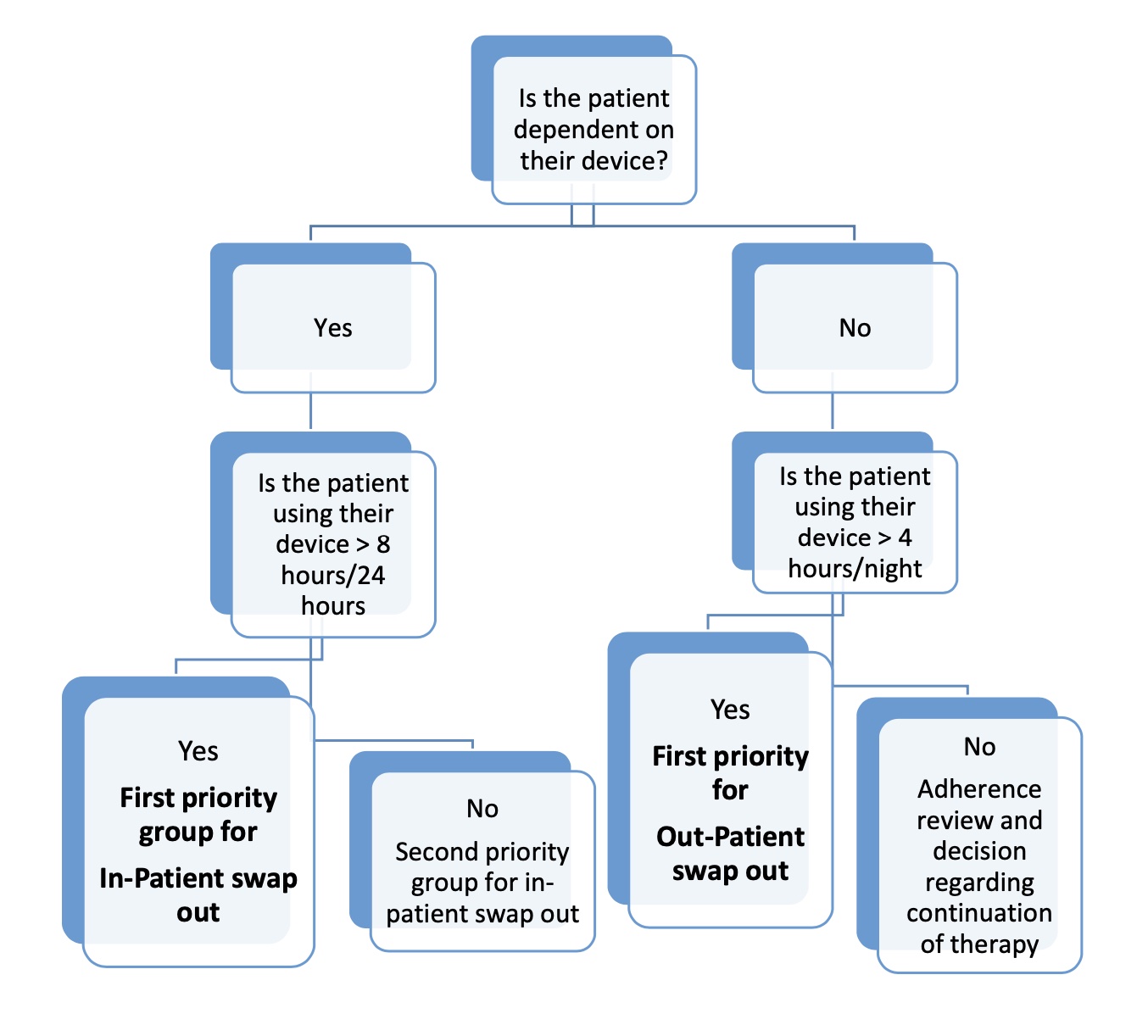
- This prioritisation of patients considers the different patient groups’ specific clinical needs and other relevant factors e.g. device dependency.
- Paediatric patients have been designated as first & high priority.
- The replacement/ repair process will be undertaken by the relevant supplier/distributor in conjunction with the patient’s primary paediatric respiratory team.
- Prior to commencement of the replacement/repair process a risk assessment & stratification is recommended to be performed by the primary paediatric respiratory team – see below the risk assessment & stratification for paediatric patients.
- Decision making regarding treatment continuation or cessation should be made jointly with the patient and their primary caregiver.
Risk Assessment & Stratification for Paediatric Patients:
- This assessment needs to take into account:
- the severity of the underlying disease
- adherence to therapy as assessed by an up to date download of usage data fromtheir current device
- severity of daytime dysfunction without treatment
- any evidence of acute or chronic symptoms of foam toxicity
- local suppliers/distributors availability of alternative devices
- Swapping out of NIV devices must not be performed without supervision from the clinical team.
- Children stratified into the urgent dependent group should be swapped out as an in- patient where they can be:
- monitored
- have oximetry and transcutaneous CO2 monitoring performed on the new device as a minimum
- new device download reviewed
- carers trained on the new device
- In the case of children with home care packages, the carers should be trained on the new device prior to admission to avoid delays in discharge
- Children stratified into the urgent non-dependent group can be considered for out- patient swap out where:
- The care giver will be trained on new device
- Home oximetry/TCOM is performed on the new device
- Download of new device and home study is reviewed by consultant
- Ensure follow up in the paediatric respiratory clinic is scheduled.
Risk Stratification for Paediatric Patients: